First human trials of long-acting ‘PrEP-like’ drug underway
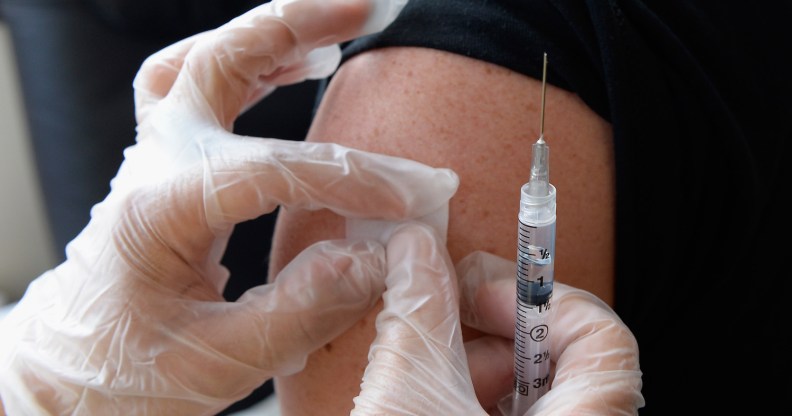
Clinical trials have begun to test the viability of a long-term drug that could see the end to a daily PReP regime.
4500 men and transgender women who have sex with men from Asia, America and Africa will take part in the study.

Trials began on December 21 and will involve researchers investigating if an injectable form of the anti-HIV drug cabotegravir can safely protect men and transgender women as well as Truvada, currently the only licensed regimen of pre-exposure prophylaxis (PrEP).
“We urgently need more HIV prevention tools that fit easily into people’s lives,” said Dr Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID).
“Although daily oral Truvada clearly works for HIV prevention, taking a daily pill while feeling healthy can be difficult for some people.
“If proven effective, injectable cabotegravir has the potential to become an acceptable, discreet and convenient alternative for HIV prevention.”
According to News Medical Life Sciences, if successful the injection would only be taken once every eight weeks.
However, results are not expected to be known for at least five years.
Recently, the NHS announced it would be launching a new trial of PrEP to 10,000 men living in England and Wales.

