Monkeypox: Moderna looking into new vaccine but says virus lacks same ‘urgency’ as COVID
Pressure is growing on health officials to secure more monkeypox vaccines. (Mario Tama/Getty Images)
Pressure is growing on health officials to secure more monkeypox vaccines. (Mario Tama/Getty Images)
Moderna is considering creating a monkeypox vaccine – but admitted there isn’t the same “urgency” to it as coronavirus had.
One of the top pharmaceutical companies in the world said Wednesday (3 August) that it has launched a research initiative into seeing whether a jab could be made using mRNA technology.
“We’re obviously very aware of the monkeypox concern and obviously very sensitive to recent announcements,” Moderna president Stephen Hoge said during an investor call.
An mRNA vaccine involves using the tiny genetic molecules that tell cells to make proteins. Scientists can use this to trick the cell into making pieces of a virus instead to strengthen the immune system. This was one of the main tools researchers used to help develop the COVID-19 vaccine.
Hoge added: “We did initiate a research program. We are tracking that very closely and obviously, given the recent public health announcements and increasing concern about availability of vaccine supply, we are beginning to look at what it would take for us to use our platform and to provide a monkeypox vaccine – both [to] intervene in the current and the current epidemic but also to try and address long-term issues of supply in this public health threat.”
But Moderna CEO Stéphane Bancel said the company will for the time being divert most of its resources into the coronavirus and the flu.
“We don’t have the urgency we had when COVID happened, because as you know, there is already a vaccine on the market,” she said.
“This is not an airborne virus. I’m not aware of any scientists that believe it can get to a pandemic like COVID.”
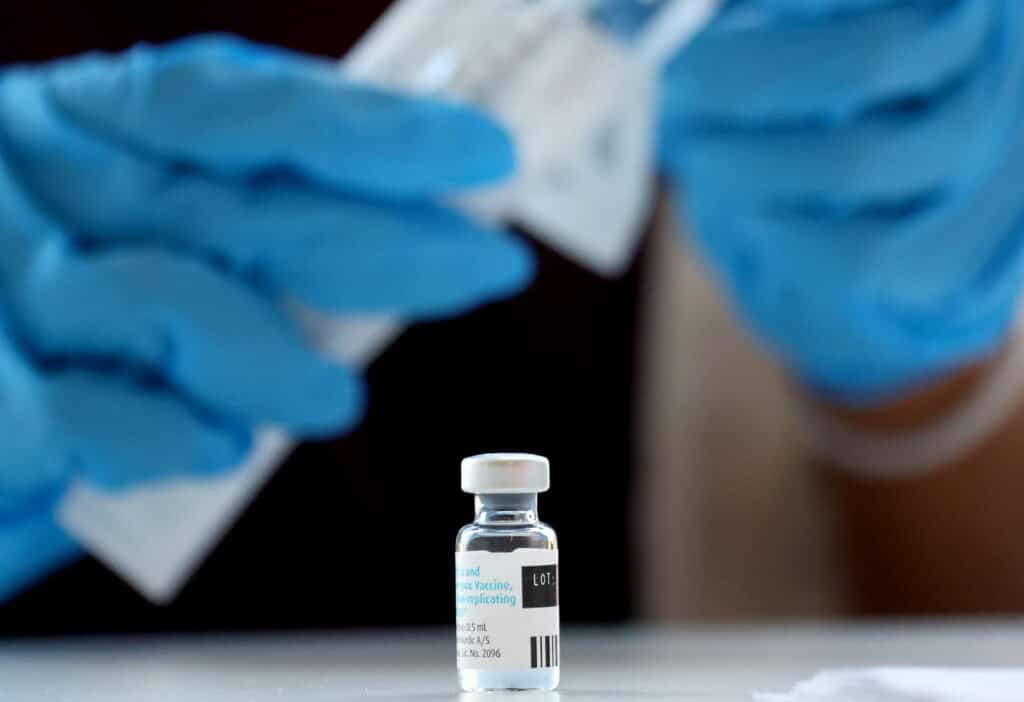
The Jynneos monkeypox vaccine, one of two jabs available, remains scarce in some US states. (Mario Tama/Getty Images)
Moderna is discussing the potential shot with the Food and Drugs Administration (FDA) and global public health regulators, Hoge added.
To get the FDA’s approval for a monkeypox vaccine, Moderna must show the agency the results of clinical trials. But Moderna’s monkeypox shot remains very much in the pre-clinical stage.
If the tides change, however, Moderna could turbocharge monkeypox jab trials.
“If we were to go after a monkeypox clinical development program, it would be to very quickly progress toward an approvable set of endpoints in a clinical study,” Hoge added.
In the meantime, the company will continue to “track” the outbreak closely and focus energies on rolling out COVID-19 booster shots, Hoge said.
The go-to vaccine for battling the new wave of monkeypox infections is Jynneos, made by Dutch company Bavarian Nordic. US federal health officials have a larger supply of ACAM2000, another FDA-approved vaccine, but it cannot be used on vulnerable people.
The Joe Biden administration has ordered nearly seven million Jynneos doses that will arrive in the coming months.
But for now, US states are struggling to dish out enough jabs. Though Jynneos is recommended to be administered in two doses 28 days apart, some cities such as New York City and Washington DC are holding back from giving out a second dose as supplies are simply too low and demand too high.
Healthcare workers and queer men and men who have sex with men, whom monkeypox has disproportionally afflicted, are currently the two main groups federal health officials are urging to get the monkeypox vaccine.
The vaccines have proved so hard to come by in some parts of the US that residents have driven hundreds of miles up to Canada to get vaccinated.
As of Wednesday (3 August), there are at least 6,600 confirmed cases of monkeypox, according to the Centers for Disease Control and Prevention.

